Implementation of tailored exercise programs for MG patients in a gym setting: a pragmatic feasibility case study
Source : https://www.nmd-journal.com/article/S0960-8966(23)00029-9/fulltext
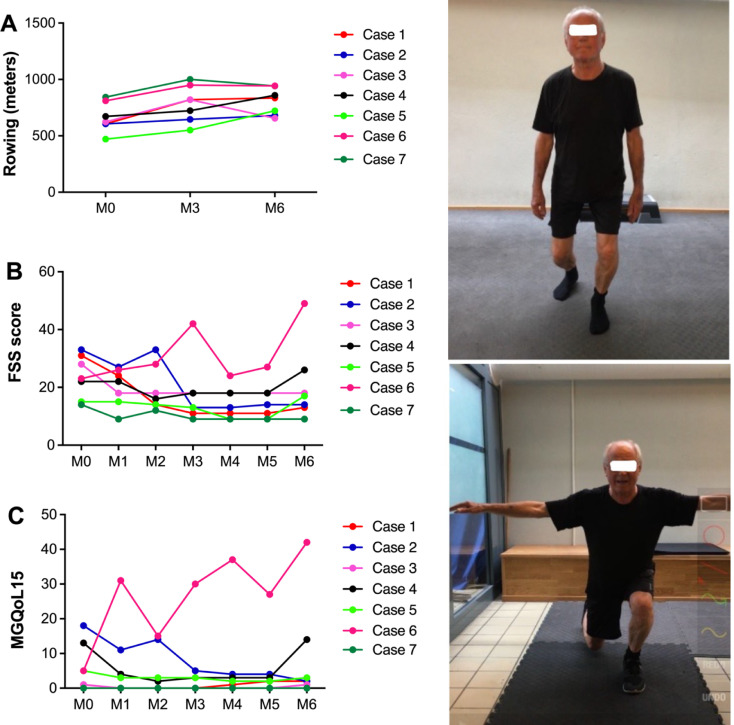
Myasthenia gravis (MG) is an autoimmune disorder where abnormal neuromuscular transmission results in fluctuating skeletal muscle weakness and fatigue, which can be worsened by physical exercise. However, based on several...
Conclusions/Relevance: Our pragmatic open-label case study suggests that well-controlled patients with generalized MG can extend their physical exercise to personal training in the gym. This is an essential step towards reducing the barriers to implementing exercise procols and increasing the availability of these interventions to MG...

Clinicoserological insights into patients with immune checkpoint inhibitor-induced myasthenia gravis - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36924454/
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure....
Conclusions/Relevance: The contrasting properties of AChR autoantibodies in these cases challenge the accuracy of serological testing in establishing definite ICI-MG diagnoses and underscore the importance of a thorough clinical assessment when evaluating ICI-related adverse events.

Facilities, selection, outcome measurement, and limitations of therapeutic plasma exchange for neuroimmunological disorders: The South East Asian survey study - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36896493/
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure....
Conclusion: Although countrywise differences exist, all share similarities regarding methods, indications, timing, obstacles, and challenges of TPE for neuroimmunological conditions. Regional collaboration will be essential to identify strategies to reduce these barriers to access to TPE in the future.

Different monoclonal antibodies and immunosuppressants administration in patients with neuromyelitis optica spectrum disorder: a Bayesian network meta-analysis - Journal of Neurology
Source : https://link.springer.com/article/10.1007/s00415-023-11641-1
Background A variety of novel monoclonal antibodies and immunosuppressant have been proved effective in treating Neuromyelitis Optica Spectrum Disorder (NMOSD). This network meta-analysis compared and ranked the efficacy and tolerability...
Conclusion: RTX and tocilizumab showed better efficacy than traditional immunosuppressants in reducing relapse. For safety, MMF and RTX had fewer AEs. However, studies with larger sample size on newly developed monoclonal antibodies are warranted in the future.

Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis - Journal of Neurology
Source : https://link.springer.com/article/10.1007/s00415-023-11617-1
Introduction The terminal complement C5 inhibitor ravulizumab has a long elimination half-life, allowing maintenance dosing every 8 weeks. In the 26-week, double-blind, randomized, placebo-controlled period (RCP) of the CHAMPION MG...
Conclusions: PK/PD evidence supports the use of ravulizumab every 8 weeks for immediate, complete, and sustained inhibition of terminal complement C5 in adults with AChR Ab+ gMG.

